Ch4 Polar Or Nonpolar - Is Ch4 Polar Or Nonpolar, Solved A Nonpolar Molecule With ... - Ch4 and ccl4 have cancelling dipole moments and so are not polar.
Ch4 Polar Or Nonpolar - Is Ch4 Polar Or Nonpolar, Solved A Nonpolar Molecule With ... - Ch4 and ccl4 have cancelling dipole moments and so are not polar.. Is co2 polar or nonpolar? Choose from 500 different sets of flashcards about polar nonpolar on quizlet. Is brcl3 polar or nonpolar? Ch4 polar mı apolar mı? 4 hydrogen atoms connected tetrahedrally with a.
Hey guys in this video we are going to determine the polarity of methane having a chemical formula of ch4to know the examples would be carbon dioxide oco and methane ch4. I think a good way to solve these in general is to first draw the molecule and. Polar protic vs polar aprotic vs nonpolar: In chemistry, polarity refers to the distribution of electric charge around atoms, chemical groups, or molecules. Depending on the relative electronegativities of the two atoms sharing electrons, there may be partial transfer of electron density nonpolar covalent bonds, with equal sharing of the bond electrons, arise when the electronegativities of the two atoms are equal.

.are used up, it is a non polar molecule.
Polar protic vs polar aprotic vs nonpolar: There are, however, c2h2 and ch4, both of which are nonpolar. 4 hydrogen atoms connected tetrahedrally with a. The polarity of the molecule depends on many factors like the shape and the components of the elements. Is ch4 polar or nonpolar. Feel free to request other molecules that i should be posting about. A lot of students i talk to have questions about solvents, so i've these solvents have moderately higher dielectric constants than the nonpolar solvents (between 5 and 20). People are now accustomed to using the. In today's video, i determine whether methane (ch4) is polar or nonpolar. Is ch4 polar or nonpolar. The molecules that have atoms with equal electronegativity are nonpolar in nature because the equal charge why is ch4 nonpolar? Ch4 polar mı apolar mı? In chemistry, polarity refers to the distribution of electric charge around atoms, chemical groups, or molecules.
About solvents in organic chemistry. Is ch 4 polar or nonpolar? Ch4 is nonpolar because all of the nonpolar covalent bonds are spaced within a tetrahedral structure around the molecule. Draw the lewis structure first). I'll tell you the polar or nonpolar list below.
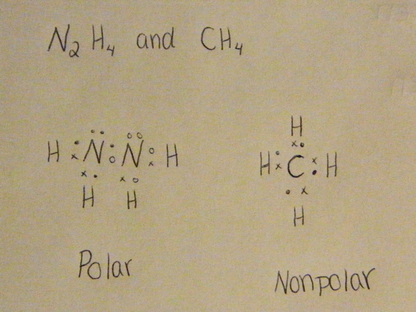
4 hydrogen atoms connected tetrahedrally with a.
In chemistry, polarity refers to the distribution of electric charge around atoms, chemical groups, or molecules. Exemples:ch4 there are four bonding pairs, and no lone pairs, that means all of the valence. Polar protic vs polar aprotic vs nonpolar: Ch4 contains nonpolar covalent bonds because the electronegativity difference between hydrogen. Feel free to request other molecules that i should be posting about. Polar molecules vs nonpolar molecules. Is brcl3 polar or nonpolar? The polarity of the molecule depends on many factors like the shape and the components of the elements. Methane ch 4 is a non polar hydrocarbon compound composed out of a single carbon atom and 4 hydrogen atoms. Decide if the following molecules are polar or nonpolar. Learn about polar nonpolar with free interactive flashcards. Polar and nonpolar molecules are the two broad classes of molecules. Ch4 and ccl4 have cancelling dipole moments and so are not polar.
Submit answer retry entire group 9 mo. People are now accustomed to using the. So, first off, methane (ch₄) is nonpolar because its c—h bonds do not have great enough of an electronegativity (en) difference for the bond to be a polar bond requires a significant en difference, so that the electrons are significantly drawn towards one atom and away from the other; Is co2 polar or nonpolar? Since they have intermediate polarity they are good.
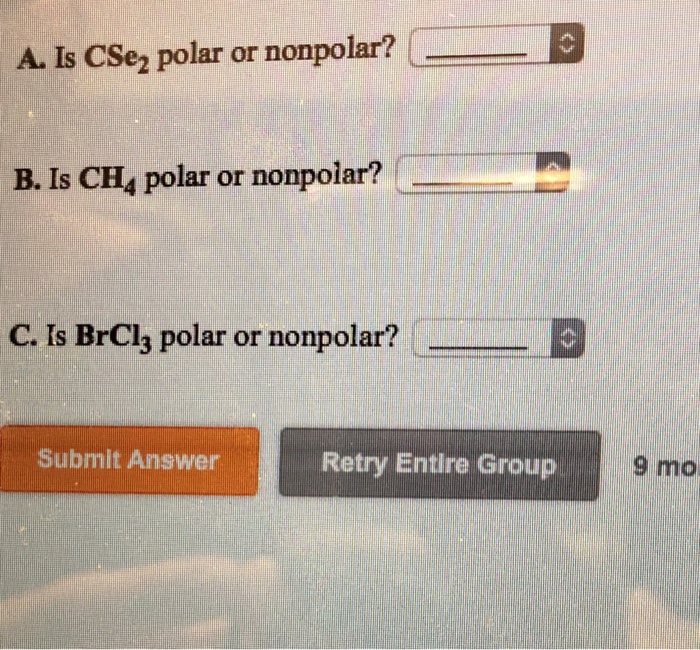
Is ch2 polar or non polar?
You can also refer to the ch4 lewis structure first to understand the arrangement of atoms in the molecule along with its properties. Is ch 4 polar or nonpolar? I'll tell you the polar or nonpolar list below. If you want to quickly find the word you want to search, use ctrl + f, then type the word you want to search. Determine if silicon tetrachloride sicl4 is polar or nonpolar. In today's video, i determine whether methane (ch4) is polar or nonpolar. Nh3 is polar for the same reason as water. Is brcl3 polar or nonpolar? Nonpolar molecules occur when electrons are shared equal between atoms of a diatomic molecule or when polar bonds in a larger molecule cancel each. Submit answer retry entire gr. So, first off, methane (ch₄) is nonpolar because its c—h bonds do not have great enough of an electronegativity (en) difference for the bond to be a polar bond requires a significant en difference, so that the electrons are significantly drawn towards one atom and away from the other; Molecular polarity (polar or nonpolar). Polarity polar nonpolar polar polar polar.
Komentar
Posting Komentar